|
Back to Annual Meeting Program
Chimeric Antibodies to CEA Improve Detection of Human Colon Cancer in Orthotopic Mouse Models
Cristina a. Metildi*1, Sharmeela Kaushal1, George a. Luiken2, Mark a. Talamini1, Robert M. Hoffman1,3, Michael Bouvet1
1Surgery, University of California San Diego, La Jolla, CA; 2OncoFluor, San Diego, CA; 3AntiCancer, Inc., San Diego, CA
Positive surgical margins after colorectal cancer surgery are strong predictors for higher local recurrence rates and poor overall survival. Currently, no real-time, reliable detection assays for positive surgical margins at the time of surgery exist. We have previously shown improved detection and resection of primary pancreatic cancer with a mouse-derived fluorophore-conjugated antibody against the tumor antigen CEA in open laparotomies in mouse models. The aim of this study was to demonstrate improved sensitivity and specificity of a new chimerized antibody against CEA in detection of CEA-expressing colon cancer for improved resection in xenograft mouse models.
Mouse models of human colon cancer were established with fragments of a CEA-expressing patient colon tumor. Two to four weeks after implantation, mice were randomized to fluorescence-guided surgery (FGS) or bright-field surgery (BS). Mice in the FGS group received tail vein injections of the chimeric anti-CEA-Alexa-488 antibody 24 hours prior to resection. Pre- and postoperative images were obtained to assess for completeness of resection. Mice were then followed for 6 months postoperatively to assess for recurrence and overall survival. At termination, all tumor lesions were harvested and evaluated histologically. The chimeric antibody was also tested on frozen tumor and normal tissue arrays comparing it to the mouse antibody.
The chimeric antibody directed against CEA demonstrated improved sensitivity and specificity in labeling CEA-expressing tumor compared to the mouse antibody. The fluorophore conjugation efficiency to the chimeric CEA antibody (Chi-Ab) was 2-fold higher than the mouse CEA antibody (M-Ab). On frozen tumor tissue arrays, the signal intensity of the Chi-Ab was significantly brighter compared to the M-Ab indicating improved binding to tumor tissue. For colon, pancreas and lung tumor samples, the signal intensity with the Chi-Ab was 94.1, 85.3, and 106.1, respectively compared to 72.5, 60.1, and 80.5 for M-Ab (p<0.001). The Chi-Ab demonstrated improved specificity as evident by the lower signal intensity in normal human tissue samples compared to M-Ab (normal colon tissue: 4.3 vs 5.4; normal pancreas tissue: 1.5 vs 2.7) indicating decreased binding of Chi-Ab. The chimeric CEA antibody was also accurate in labeling human colon cancer in mouse xenografts enabling improved detection of tumor margins for more effective resection, increasing the R0 resection rate from 86% to 96%.
The chimeric form of our fluorophore-conjugated CEA antibody has more effective labeling of human CEA-expressing cancer in tissue arrays and in our xenograft mouse models of human colon cancer. The improved sensitivity and specificity of the chimeric fluorophore-conjugated antibody is clinically translatable.
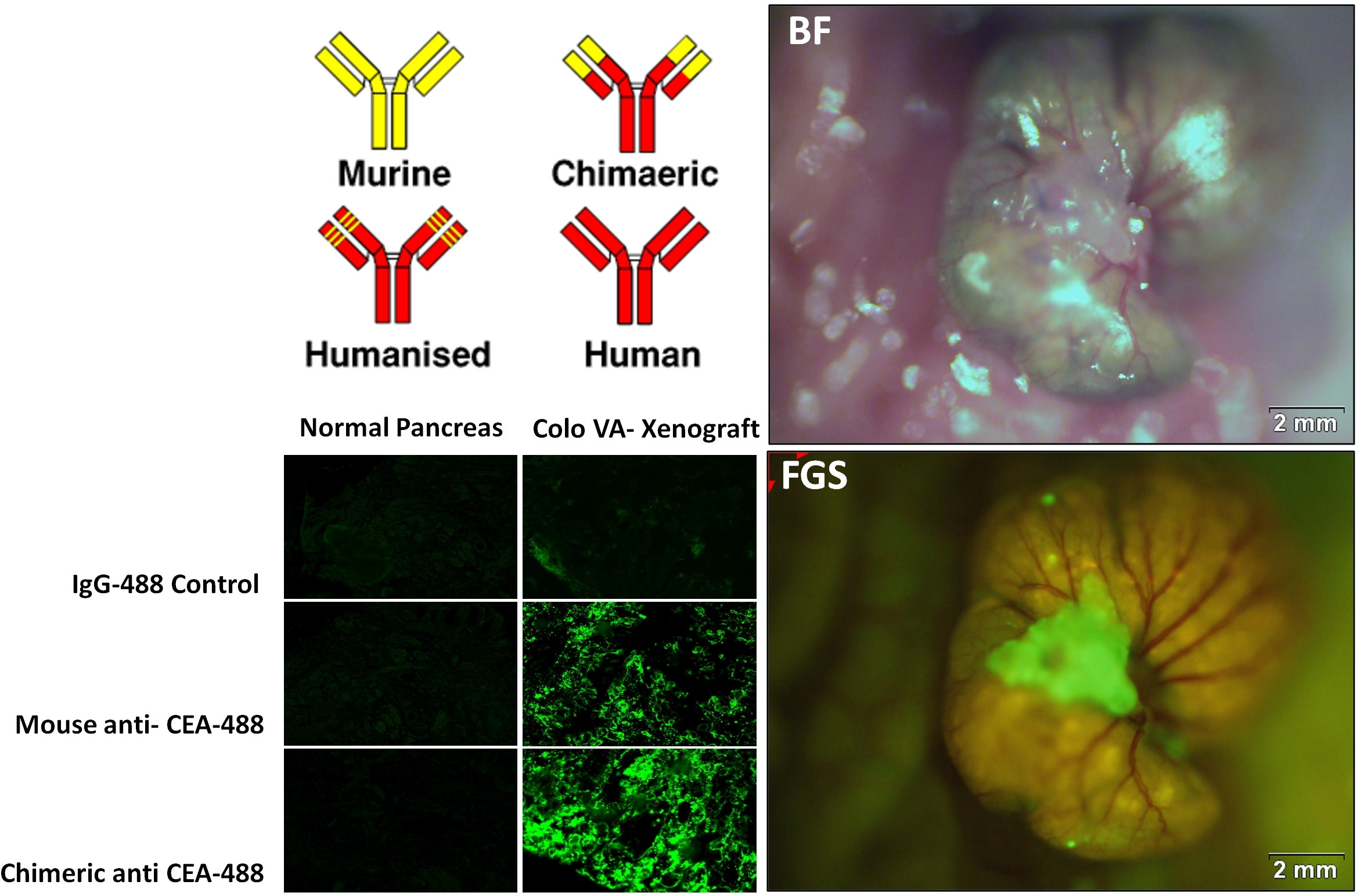
The top left panel is an illustration of the steps required to convert a mouse antibody to a human antibody. Before fully humanizing the antibody, we tested the chimeric antibody on normal tissue and CEA-expressing colon tissue samples, comparing its labeling sensitivity and specificity to the mouse antibody. The bottom left panel shows that a brighter signal is obtained by labeling the tumor with the chimeric antibody, as compared to the mouse antibody. Also, there is less labeling on normal tissue with the chimeric antibody. The two panels on the right illustrate the improved detection of CEA-expressing colon tumor in our mouse models with the chimeric antibody.
Back to Annual Meeting Program
|


