# 104191 Abstract ID: 104191 Restoring Apoptosis in Pancreatic Cancer Cells by Targeting the NF-?b Signaling Pathway with Anti-EGFR Antibody
Guido M Sclabas, Shuichi Fujioka, Christian Schmidt, Zhen Fan, Douglas B Evans, Paul J Chiao, Houston, TX
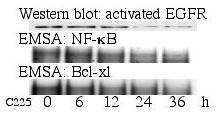
Purpose: To study the effect of Epidermal Growth Factor Receptor (EGFR) on the NF-?B signaling pathway and apoptosis in human pancreatic adenocarcinoma. EGFR and Bcl-xl are overexpressed and RelA/ NF-?B constitutively activated in the majority of human pancreatic cancer cell lines and tissue specimens. Work from our laboratory has suggested that these factors may contribute to the resistance of pancreatic cancer cells to chemotherapy induced apoptosis. Therefore, in the present study we blocked the EGFR with the specific monoclonal chimeric antibody C225. Method: Human pancreatic cancer cell line MDAPanc-28 was cultured in vitro and treated with C225 to specifically analyze its effect on RelA/ NF-?B -, Bcl-2 family members-, AP-1- and p53 levels, using western and northern blot analysis, EMSA, RNase protection assay and cell proliferation assay. Results: Activated EGFR was markedly decreased in western-blot analysis after 12 hours of incubation with C225 and no longer detectable after 24 hours, while total EGFR expression remained unchanged. A decrease in NF-?B DNA binding activity was also found in the nuclear fraction of cellular protein extract. In the same fraction a decrease in Bcl-xl and AP-1 was observed, corresponding to the NF-?B DNA binding activity. p53 DNA binding activity was also downregulated over the 36 hour incubation period. Bcl-2 family members bcl-xl and bfl-1 were also downregulated in the RNase protection assay, while no difference in p53 expression could be found on northern blot analysis. Finally, cell growth was suppressed by 30% in the cell proliferation assay. Conclusions: NF-?B and Bcl-2 family members are involved in the chemoresistence seen in human pancreatic cancer. Specifically blocking EGFR with C225 leads to decreased RelA/ NF-?B activity in pancreatic cancer cells. This restores apoptosis by suppressing expression of anti-apoptotic Bcl-xl and bfl-1, probably in a p53 independent manner. Further, AP-1 suppression may be beneficial due to its role in the EGFR activated cytoskeletal rearrangement required for cell motility and invasion. This may be one explanation for the exciting results emerging from clinical studies evaluating EGFR inhibition.
|
 500 Cummings Center
500 Cummings Center +1 978-927-8330
+1 978-927-8330
 +1 978-524-0461
+1 978-524-0461