# 104006 Abstract ID: 104006 Selective Cyclooxygenase-2 Inhibitor Rofecoxib (VioxxTM) Induces Expression of Cell Cycle Arrest Genes and Slows Tumor Growth in Human Pancreatic Cancer
William W Tseng, May N Chen, Adriana Deganutti, Romaine E Saxton, Carson D Liu, Chicago, IL; Los Angeles, CA
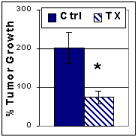
Recent studies have indicated that cyclooxygenase-2 (COX-2) is over-expressed in pancreatic adenocarcinoma and may play a critical role in this rapidly progressing form of cancer. PURPOSE: To evaluate the effects of an FDA-approved, selective COX-2 inhibitor on pancreatic cancer cell gene expression and in vivo growth. METHODS: A human pancreatic adenocarcinoma cell line, MiaPaCa-2 was incubated for 18 hrs with 5 microM of rofecoxib (VioxxTM, Merck) for in vitro studies. mRNA was isolated and gene expression analyzed by cDNA microarray chips. For in vivo studies, athymic mice were orthotopically injected with 7.5 x 10^5 MiaPaCa-2 cells through a mini-laparotomy. After 1 month, mice were reoperated and randomized to receive either 12.5 mg/kg/day of rofecoxib in reformulated rodent chow (n=9) or reformulated rodent chow alone (n=6) for 21 days. Tumor growth was assessed by comparing tumor volume before and after treatment. Data was expressed as the mean +/- SEM and statistical analyses were performed using a Student's t-test. RESULTS: Rofecoxib decreased gene expression of cyclin D1/PRAD1, a key component of cell cycle progression, while increasing expression of several cell cycle arrest genes, including p21/WAF1, p33/ING, GADD34, and GADD45. (p<0.05) Tumor growth was reduced by 2.7 fold in treated versus control mice. (*p<0.05) Grossly, tumors in treated mice were not as well vascularized or locally-invasive compared to control tumors. No toxicity was observed in mice receiving rofecoxib, based on body weight obtained during treatment. CONCLUSIONS: Rofecoxib, a selective COX-2 inhibitor, slows growth of human pancreatic cancer through changes in gene expression that favor cell cycle arrest. Rofecoxib may have clinical utility in the management of patients found to have unresectable or metastatic pancreatic cancer at the time of operation.
|
 500 Cummings Center
500 Cummings Center +1 978-927-8330
+1 978-927-8330
 +1 978-524-0461
+1 978-524-0461