# 102566 Abstract ID: 102566 Mucosal Healing in Crohn's Disease Patients Is Associated with Reduction in Hospitalizations and Surgeries
Paul Rutgeerts, Helmut Malchow, Morten H Vatn, Songkai Yan, Mohan Bala, Sander Van Deventer, Leuven, Belgium; Leverkusen, Germany; Oslo, Norway; Malvern, PA; Amsterdam, The Netherlands
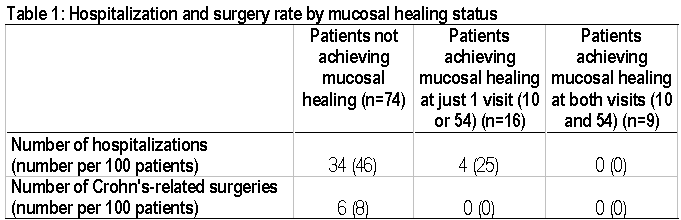
Background: The relationship between mucosal healing and rates of hospitalizations and surgeries in Crohn's disease patients has not been studied. Methods: In the ACCENT I trial 573 patients with a CDAI between 220 and 400 received a single infusion of 5 mg/kg infliximab. At week 2, responders and non-responders were separately randomized to 1 of 3 maintenance regimens: 1) placebo at wks 2, 6 and then placebo q 8 weeks, 2) 5 mg/kg infliximab at wks 2, 6 and then 5 mg/kg q 8 weeks, and 3) 5 mg/kg infliximab at wks 2, 6 and then 10 mg/kg infliximab q 8 weeks. Starting at week 14, patients who lost response were allowed to crossover to episodic treatment with infliximab at a dose 5mg/kg higher than their assigned maintenance dose. All patients enrolling at 25 selected sites were eligible to participate in the mucosal healing substudy. Endoscopic assessments were done at weeks 0, 10 and 54. Mucosal healing was defined as the absence of mucosal ulceration. Analysis at 54 weeks included data subsequent to crossover. Results: A total of 99 patients participated in the mucosal healing substudy. Table 1 shows the number of hospitalizations and Crohn's-related surgeries in patients achieving mucosal healing at neither week 10 and week 54, at just one of these visits, and at both of these visits. Conclusions: Crohn's disease patients not achieving mucosal healing are at a much higher risk of being hospitalized and requiring surgery. Healing the mucosa has the potential to prevent hospitalizations and surgeries in these patients.
|
 500 Cummings Center
500 Cummings Center +1 978-927-8330
+1 978-927-8330
 +1 978-524-0461
+1 978-524-0461