# 101681 Abstract ID: 101681 Epidermal Growth Factor Activation of Intestinal Glutamine Transport Is Mediated by Mitogen-Activated Protein Kinases
Christopher L Wolfgang, Wiley W Souba Jr, Chengmao Lin, Qinghe Meng, Anne M Karinch, Ming Pan, Hershey, PA
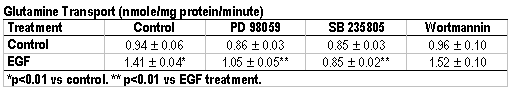
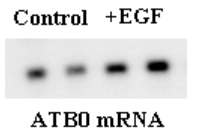
Background: Glutamine is an essential nutrient for gut functions but the regulation of its uptake by intestinal mucosal cells is poorly understood. Given the pivotal role of epidermal growth factor (EGF) in regulating gut metabolism, growth and differentiation, the purpose of this in vitro study was to investigate the intracellular signaling pathways involved in the regulation of EGF-mediated intestinal glutamine transport. Methods: 3H-glutamine (50 µM) transport activity and mRNA levels for the intestinal glutamine transporter ATB0 were measured in cultured intestinal epithelial Caco-2 cells. Cells were treated with EGF (0 - 100 ng/ml), the mitogen-activated protein kinase (MAPK) MEK1 inhibitor PD 98059 (50 µM), the p38 MAPK inhibitor SB 235805 (10 µM), and the Phosphatidylinositide-3 kinase (PI-3) inhibitor wortmannin (10 µM). Data were analyzed by ANOVA. Results: Prolong incubation of EGF (48 hours) resulted in an 80% increase in glutamine transport gene ATB0 mRNA levels (1.82 ? 0.10 vs 1.0 ? 0.11, p < 0.01) (see figure) and a 50% increase in glutamine transport activity (see table). This EGF-induced glutamine transport activity was blocked individually by PD 98059 and SB 235805, but not by wortmannin (see table). Glutamine transport activity in cells treated with PD 98059, SB 235805, or wortmannin alone did not differ from control cells treated with vehicle. Conclusions: EGF-activated glutamine transport in Caco-2 cells occurs via signaling pathways that lead to transcription of the glutamine transporter gene. Mitogen-activated protein kinases MEK and p38, but not PI-3, are key intracellular regulators involved in this signal transduction cascade.
|
 500 Cummings Center
500 Cummings Center +1 978-927-8330
+1 978-927-8330
 +1 978-524-0461
+1 978-524-0461