Introduction: Since TNF-a and some chemotherapeutic agents activate both apoptosis and NF-kB dependent anti-apoptotic genes, they may neutralize their own anti-tumor effects. The cell signaling mechanisms for such chemoresistance are not clear, but may involve PI-3'Kinase (PI3K). To clarify this, we examined whether cross-signaling between PI3K and NF-kB enhances the anti-tumor effect of TNF-a in human pancreatic cancer cells.
Methods: Quiescent pancreatic cancer cells (Panc-1, MiaPaCa-2)with TNF-a, Ly294002 (PI3K inhibitor), alone or combined, were restimulated with mitogen (10% FCS) to induce cell cycle entry. Proliferation (MTT), cell cycle progression (ApoBrDU incorporation and FACS analysis), and apoptosis (PARP cleavage; caspase-3 activation - Western blot [WB])were measured. Akt activation (Akt kinase assay) and IkBa degradation were determined by WB. Translocation of NF-kB into the nucleus was examined by EMSA, while a NF-kB/luciferase reporter gene was used to quantify NF-kB dependent gene expression. Statistical analysis - two-tailed t-test (p<0.05).
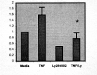
Results: PI3K inhibition significantly enhanced the anti-proliferative and pro-apoptotic effects of TNF-a in both cell lines, but only after arresting cells in G0-G1 phase. Ly294002 also blocked TNF-a-induced Akt activation, but failed to alter cytoplasmic IkBa degradation or subsequent NF-kB nuclear translocation. NF-kB dependent gene expression, however, was ultimately suppressed by Ly294002 (Fig 1), suggesting that PI3K-dependent activation of NF-kB is IkBa-independent.
Conclusions: PI3K inhibition can block NF-kB dependent gene expression regardless of cytoplasmic IkBa/NF-kB activation. Since it also regulates the anti-tumor effects of TNF-a, PI3K may in part determine NF-kB induced chemoresistance in human pancreatic cancer.
 500 Cummings Center
500 Cummings Center +1 978-927-8330
+1 978-927-8330
 +1 978-524-0461
+1 978-524-0461