Swelling by hypoosmotic stress activates PI-3-K, but its impact on the downstream signal PKB or cell growth is unknown. Although AP-1 activation is in part PI-3-K-dependent, its response to hypoosmotic stress is also undefined. We hypothesized that cell swelling modulates proliferation in HepG2 cells via PKB-dependent activation of AP-1.
Methods: HepG2 incubated for 1 hr with/out 50 mM LY294002 (LY), a PI-3-K inhibitor, were exposed for up to 30 min to hypoosmotic medium (200mOsm/L) to induce swelling. TNF-a (1.4 nM) or normoosmotic medium served as positive and negative controls, respectively. Western blots measured cytoplasmic phospho- and total PKB. EMSA measured nuclear AP-1. Methylene blue assays measured cell proliferation at 24, 48 and 72 hrs after pulse exposure to hypoosmotic stress. T test and ANOVA verified statistical significance. All experiments were performed in triplicate.
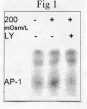
Results: [1] Hypoosmotic stress phosphorylated PKB (P-PKB) by 10 min, and this effect was inhibited by LY. [2] Hypooosmotic stress translocated AP-1 by 30 min, and this effect was inhibited by LY (Fig 1). [3] Hypoosmotic stress potentiated growth by 72 hrs as compared to both control and LY-inhibited cells (n=4 per group, p=0.009# and p=0.004*, respectively; p<0.001, ANOVA).
Conclusion: In HepG2 cells, hypoosmotic stress-induced swelling acts as a proliferative signal which is mediated by the PKB-dependent activation of AP-1. These data delineate a possible mechanism linking cell volume change to growth in human liver cancer.
 500 Cummings Center
500 Cummings Center +1 978-927-8330
+1 978-927-8330
 +1 978-524-0461
+1 978-524-0461